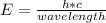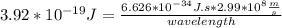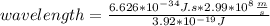Chemistry, 20.08.2020 01:01 mscharris66
The equation for photon energy, E, is E=hc/λ
where h = 6.626×10−34 J⋅s (Planck's constant) and c = 2.99×108 m/s (the speed of light).
What is the wavelength, λ, of a photon that has an energy of E = 3.92×10−19 J ?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
The wilson chamber is used to study: direction, speed, and distance of radioactivity the intensity of radiation the appearance of individual atoms all of the above
Answers: 1

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
The equation for photon energy, E, is E=hc/λ
where h = 6.626×10−34 J⋅s (Planck's constant) and c =...
Questions

Business, 14.04.2021 19:20

Chemistry, 14.04.2021 19:20



History, 14.04.2021 19:20

Social Studies, 14.04.2021 19:20

English, 14.04.2021 19:20


Biology, 14.04.2021 19:20

Mathematics, 14.04.2021 19:20

English, 14.04.2021 19:20

Chemistry, 14.04.2021 19:20

Mathematics, 14.04.2021 19:20


Mathematics, 14.04.2021 19:20

English, 14.04.2021 19:20