
Chemistry, 19.08.2020 02:01 markuswalter1043
A student made a graph to show the chemical equilibrium position of a reaction.
The student forgot to label the y-axis of the graph.
What best explains the label that the student should use on the y-axis? A. Concentration, because as the amount of product decreases, the amount of reactant increases over time.
B. Reaction rate, as the rates of forward and backward reactions become equal at equilibrium.
C. Concentration, because the amounts of reactants and products remain constant after equilibrium is reached.
D. Reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
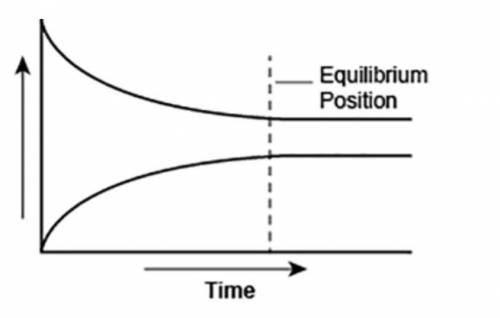

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
A student made a graph to show the chemical equilibrium position of a reaction.
The student forgot...
Questions

Mathematics, 14.12.2020 21:00


Mathematics, 14.12.2020 21:00


Mathematics, 14.12.2020 21:00

Biology, 14.12.2020 21:00


Chemistry, 14.12.2020 21:00

Mathematics, 14.12.2020 21:00


Mathematics, 14.12.2020 21:00

Mathematics, 14.12.2020 21:00

Mathematics, 14.12.2020 21:00




Arts, 14.12.2020 21:00


Mathematics, 14.12.2020 21:00

Mathematics, 14.12.2020 21:00



