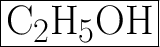Chemistry, 18.08.2020 14:01 bigmouth804
The following compounds have similar molecular weights. Which has the highest boiling point?
A. CH3OCH3
B. C2H5OH
C. CH3CH2CH3
D. CH3CH=O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2

Chemistry, 23.06.2019 11:00
The decimals you found in part b are called repeating decimals. in the gizmo, repeating decimals are rounded to two places. how does the gizmo show you that a decimal has been rounded?
Answers: 3

Chemistry, 23.06.2019 22:00
How will the ice water mix with the salty water? write your hypothesis below. solution hypothesis describing mixing hypothesis describing temperature (will the top be warmer, cooler, or the same as the bottom? ) solution 1 freshwater solution 2 salty water solution 3 saturated water?
Answers: 1
You know the right answer?
The following compounds have similar molecular weights. Which has the highest boiling point?
A. CH3...
Questions

Mathematics, 24.02.2021 09:40

Advanced Placement (AP), 24.02.2021 09:40

History, 24.02.2021 09:40


English, 24.02.2021 09:40

Geography, 24.02.2021 09:40


Mathematics, 24.02.2021 09:40


English, 24.02.2021 09:40

Social Studies, 24.02.2021 09:40


Mathematics, 24.02.2021 09:40

World Languages, 24.02.2021 09:40

Mathematics, 24.02.2021 09:40


Health, 24.02.2021 09:40

Social Studies, 24.02.2021 09:40

Biology, 24.02.2021 09:40