Chemistry, 18.08.2020 22:01 superbatman9193
For the reaction of hydrochloric acid and sodium thiosulfate, the rate law is written as:
A. Rate = k [HCl]m [Na2S2O3]n
B. Rate = k [HCl + Na2S2O3]
C. Rate = t [HCl]a [Na2S2O3]x
D. Rate = t [HCl + Na2S2O3]

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:10
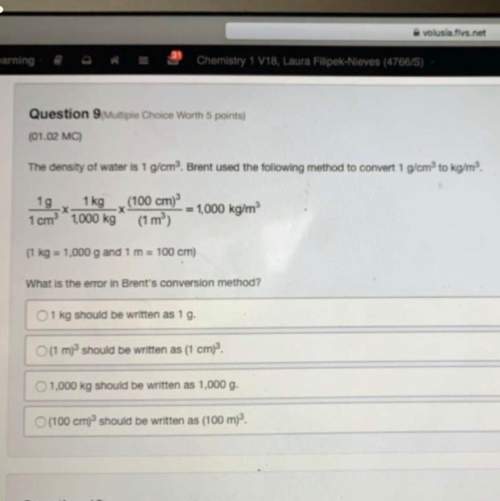
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1


Chemistry, 23.06.2019 15:00
Food can lose electrons to air. this loss of electrons can create free radicals that destroy chemical bonds, hence spoiling the food. which term describes this process?
Answers: 1
You know the right answer?
For the reaction of hydrochloric acid and sodium thiosulfate, the rate law is written as:
A. Rate =...
Questions

English, 21.12.2020 07:50


Mathematics, 21.12.2020 07:50


Mathematics, 21.12.2020 07:50




History, 21.12.2020 07:50

English, 21.12.2020 07:50

Mathematics, 21.12.2020 07:50


Chemistry, 21.12.2020 07:50



Health, 21.12.2020 08:00

History, 21.12.2020 08:00

Mathematics, 21.12.2020 08:00

English, 21.12.2020 08:00

![Rate =k[HCl]^{m} [Na_2S_2O_3]^{n}](/tpl/images/0724/1062/2c070.png)