
Chemistry, 18.08.2020 20:01 epmooneyham922
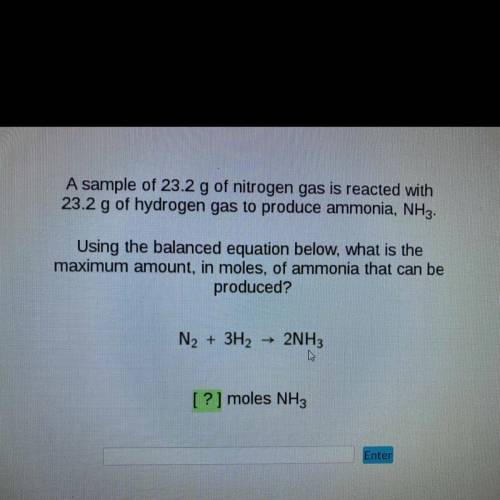
A sample of 23.2 g of nitrogen gas is reacted with
23.2 g of hydrogen gas to produce ammonia, NH3.
Using the balanced equation below, what is the
maximum amount, in moles, of ammonia that can be
produced?
N2 + 3H2
2NH3
IN
[?] moles NH3

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2


Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2
You know the right answer?
A sample of 23.2 g of nitrogen gas is reacted with
23.2 g of hydrogen gas to produce ammonia, NH3.<...
Questions

Chemistry, 26.07.2019 18:00

Social Studies, 26.07.2019 18:00


Biology, 26.07.2019 18:00

Health, 26.07.2019 18:00









Chemistry, 26.07.2019 18:00


English, 26.07.2019 18:00

History, 26.07.2019 18:00






