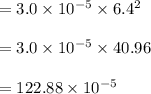Chemistry, 17.08.2020 01:01 nicholasferrell
The reaction NO2(g) + CO(g) → NO(g) + CO2(g) has been found to be second order with respect to NO2 and zero order with respect to CO. At a certain temperature, the rate constant is found experimentally to be 3.0 × 10−5 L mol · s . What is the rate of formation

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1

You know the right answer?
The reaction NO2(g) + CO(g) → NO(g) + CO2(g) has been found to be second order with respect to NO2 a...
Questions



Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30


Chemistry, 18.03.2021 01:30



Mathematics, 18.03.2021 01:30


History, 18.03.2021 01:30



Computers and Technology, 18.03.2021 01:30

Computers and Technology, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30

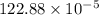 "
"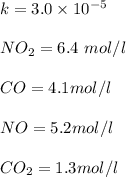

![=k.[NO_2]^2](/tpl/images/0723/2177/ff6c8.png) because the above given is the part of the second-order, which relates to
because the above given is the part of the second-order, which relates to 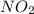 . In the zeros order the Carbon monoxide (CO) its reaction doesn't affect the rate.
. In the zeros order the Carbon monoxide (CO) its reaction doesn't affect the rate.