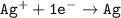Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1

Chemistry, 23.06.2019 07:10
Which one of the following is an oxidation-reduction reaction? naoh + hno3 --> h2o + kno3 naoh + hno3 --> h2o + kno3 so3 + h2o --> h2so4 cacl2 + na2co3 --> caco3 + 2 nacl ch4 + 2 o2 --> co2 + 2 h2o al2(so4)3 + 6 koh --> 2 al(oh)3 + 3 k2so4
Answers: 3
You know the right answer?
Write a chemical equation for the reaction that occurs in the following cell: Cu|Cu2+(aq)||Ag+(aq)|A...
Questions



Mathematics, 02.09.2019 00:30



Mathematics, 02.09.2019 00:30

Mathematics, 02.09.2019 00:30

Health, 02.09.2019 00:30


Arts, 02.09.2019 00:30

Social Studies, 02.09.2019 00:30



Mathematics, 02.09.2019 00:30




Health, 02.09.2019 00:30

History, 02.09.2019 00:30

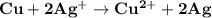
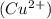 which go into the solution. The copper then becomes negatively charged and functions as the negative electrode i.e the anode
which go into the solution. The copper then becomes negatively charged and functions as the negative electrode i.e the anode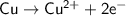
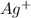 becomes reduced by gaining two electrons each from the metallic copper which was deposited into the silver electrode. The silver electrode thus becomes positively charged and functions as the positive electrode. i.e the cathode.
becomes reduced by gaining two electrons each from the metallic copper which was deposited into the silver electrode. The silver electrode thus becomes positively charged and functions as the positive electrode. i.e the cathode.