
Chemistry, 15.08.2020 23:01 holaadios222lol
Identify the formula for an alkyne. Identify the formula for an alkyne. CnH2n 4 CnH2n-2 CnH2n 2 CnH2n-4 CnH2n

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3
You know the right answer?
Identify the formula for an alkyne. Identify the formula for an alkyne. CnH2n 4 CnH2n-2 CnH2n 2 CnH2...
Questions

History, 08.12.2020 02:20

Mathematics, 08.12.2020 02:20

Mathematics, 08.12.2020 02:20

History, 08.12.2020 02:20


Chemistry, 08.12.2020 02:20

Biology, 08.12.2020 02:20


Mathematics, 08.12.2020 02:20


History, 08.12.2020 02:20


Chemistry, 08.12.2020 02:20




Mathematics, 08.12.2020 02:20


Social Studies, 08.12.2020 02:20

English, 08.12.2020 02:20

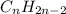
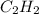 .
.

