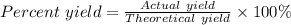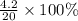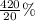Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2

Chemistry, 23.06.2019 11:40
An electron moved from a lower energy level to a higher energy level. what most likely happened during the transition? a random amount of light was released. a fixed amount of energy was absorbed. a fixed amount of energy was released. a random amount of light was absorbed.
Answers: 1
You know the right answer?
If 10.0 moles of O₂ are reacted with excess NO in the reaction below, and only 4.2 mol of NO₂ were c...
Questions



Mathematics, 25.03.2020 07:26



Mathematics, 25.03.2020 07:27

Chemistry, 25.03.2020 07:27



Mathematics, 25.03.2020 07:27


Mathematics, 25.03.2020 07:27


Computers and Technology, 25.03.2020 07:27



Mathematics, 25.03.2020 07:28


Health, 25.03.2020 07:28

Mathematics, 25.03.2020 07:28