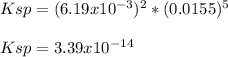Chemistry, 15.08.2020 20:01 littledogy13
The solubility of gold (V) oxalate, Au2(C2O4)5 is 2.58 g/L. Calculate Ksp from this information.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1


Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
The solubility of gold (V) oxalate, Au2(C2O4)5 is 2.58 g/L. Calculate Ksp from this information....
Questions

Business, 20.08.2019 05:30

Mathematics, 20.08.2019 05:30


Mathematics, 20.08.2019 05:30

Mathematics, 20.08.2019 05:30


Biology, 20.08.2019 05:30


Mathematics, 20.08.2019 05:30

Mathematics, 20.08.2019 05:30

Mathematics, 20.08.2019 05:30


Mathematics, 20.08.2019 05:30


Mathematics, 20.08.2019 05:30





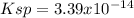
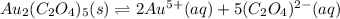
![Ksp=[Au^{5+}]^2[(C_2O_4)^{2-}]^5](/tpl/images/0722/7385/d53dd.png)
![[Au_2(C_2O_4)_5]=2.58\frac{g}{L} *\frac{1mol}{834g} =3.09M](/tpl/images/0722/7385/0abfb.png)
![[Au^{5+}]=3.09x10^{-3}\frac{mol}{L} *\frac{2molAu^{5+}}{1mol} =6.19x10^{-3}M](/tpl/images/0722/7385/94888.png)
![[(C_2O_4)^{2-}]=3.09x10^{-3}\frac{mol}{L} *\frac{5mol(C_2O_4)^{2-}}{1mol} =0.0155M](/tpl/images/0722/7385/7e2fe.png)