
Chemistry, 13.08.2020 18:01 sampurple123
Dinitrogen tetroxide and hydrazine (N2H4) undergo a redox reaction in which nitrogen and water are formed as products. What mass of nitrogen could be produced when 50.0 g of dinitrogen tetroxide and 45.0 g of hydrazine are combined?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 23.06.2019 01:00
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1
You know the right answer?
Dinitrogen tetroxide and hydrazine (N2H4) undergo a redox reaction in which nitrogen and water are f...
Questions

Mathematics, 08.07.2019 23:00

Mathematics, 08.07.2019 23:00


Mathematics, 08.07.2019 23:00



History, 08.07.2019 23:00

Mathematics, 08.07.2019 23:00

Biology, 08.07.2019 23:00


Business, 08.07.2019 23:00

Chemistry, 08.07.2019 23:00

English, 08.07.2019 23:00

English, 08.07.2019 23:00

Mathematics, 08.07.2019 23:00

Mathematics, 08.07.2019 23:00

Biology, 08.07.2019 23:00

English, 08.07.2019 23:00


Spanish, 08.07.2019 23:00

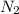 = 45.65 g
= 45.65 g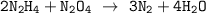
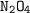 :
: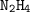 :
:

