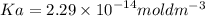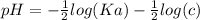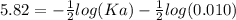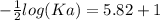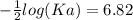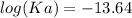Chemistry, 13.08.2020 17:01 lucifer6669
The pH of an acid solution is 5.82. Calculate the Ka for the monoprotic acid. The initial acid concentration is 0.010 M.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2
You know the right answer?
The pH of an acid solution is 5.82. Calculate the Ka for the monoprotic acid. The initial acid conce...
Questions

Mathematics, 26.06.2020 16:01

Arts, 26.06.2020 16:01




Arts, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01


Social Studies, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01


English, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01


Advanced Placement (AP), 26.06.2020 16:01

Social Studies, 26.06.2020 16:01