

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3
You know the right answer?
At 25 °C, only 0.0990 mol of the generic salt AB3 is soluble in 1.00 L of water. What is the Ksp of...
Questions


Arts, 06.11.2020 20:50

Social Studies, 06.11.2020 20:50

Mathematics, 06.11.2020 20:50

Mathematics, 06.11.2020 20:50




Chemistry, 06.11.2020 20:50


English, 06.11.2020 20:50




History, 06.11.2020 20:50



Mathematics, 06.11.2020 20:50


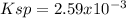
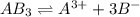
![[A]=0.099 \frac{molAB_3}{L}*\frac{1molA}{1molAB_3} =0.0099M](/tpl/images/0722/0213/dd0c8.png)
![[B]=0.099 \frac{molAB_3}{L}*\frac{3molB}{1molAB_3} =0.000.297M](/tpl/images/0722/0213/411cb.png)
![Ksp=[A][B]^3](/tpl/images/0722/0213/9740b.png)
![Ksp=[0.099M][0.297M]^3\\\\Ksp=2.59x10^{-3}](/tpl/images/0722/0213/a2275.png)


