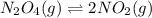Chemistry, 14.08.2020 04:01 sillslola816oxb5h7
If we represent the equilibrium as:...N2O4(g) 2 NO2(g) We can conclude that: 1. This reaction is: A. Exothermic B. Endothermic C. Neutral D. More information is needed to answer this question. 2. When the temperature is increased the equilibrium constant, K: A. Increases B. Decreases C. Remains the same D. More information is needed to answer this question. 3. When the temperature is increased the equilibrium concentration of NO2: A. Increases B. Decreases C. Remains the same D. More information is needed to answer this question.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
You know the right answer?
If we represent the equilibrium as:...N2O4(g) 2 NO2(g) We can conclude that: 1. This reaction is: A....
Questions

Chemistry, 14.11.2020 03:10


Biology, 14.11.2020 03:10


English, 14.11.2020 03:10

English, 14.11.2020 03:10

Biology, 14.11.2020 03:10




English, 14.11.2020 03:10

Mathematics, 14.11.2020 03:10

Social Studies, 14.11.2020 03:10




Biology, 14.11.2020 03:10

History, 14.11.2020 03:10

Mathematics, 14.11.2020 03:10