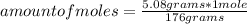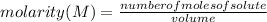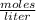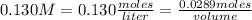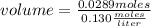Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
How many mL of a 0.130 M aqueous solution of chromium(II) nitrate, Cr(NO3)2, must be taken to obtain...
Questions

English, 15.10.2020 08:01



Business, 15.10.2020 08:01


Mathematics, 15.10.2020 08:01

English, 15.10.2020 08:01

Mathematics, 15.10.2020 08:01





Mathematics, 15.10.2020 08:01


Spanish, 15.10.2020 08:01