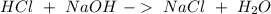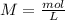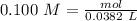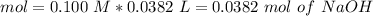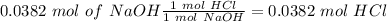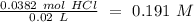Chemistry, 12.08.2020 08:01 eraines1714
4. A 0.100 M solution of NaOH is used to titrate an HCl solution of unknown concentration. To neutralize the solution, an average volume of
the titrant was 38.2 mL. The starting volume of the HCI solution was 20 ml. What's the concentration of the HCI?
O A.0.284 M
B. 3.34 M
C. 0.191 M
D. 0.788 M

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1



Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
4. A 0.100 M solution of NaOH is used to titrate an HCl solution of unknown concentration. To neutra...
Questions



Mathematics, 24.08.2021 09:30

Physics, 24.08.2021 09:30



Mathematics, 24.08.2021 09:30

Mathematics, 24.08.2021 09:30

English, 24.08.2021 09:30

Social Studies, 24.08.2021 09:30

Mathematics, 24.08.2021 09:30

Mathematics, 24.08.2021 09:40

Mathematics, 24.08.2021 09:40

Mathematics, 24.08.2021 09:50



Mathematics, 24.08.2021 09:50


Mathematics, 24.08.2021 09:50

Advanced Placement (AP), 24.08.2021 09:50