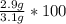Chemistry, 12.08.2020 07:01 kokokakahi
A student carries out the precipitation reaction shown below, starting with 0.030 moles of calcium nitrate. The final mass of the precipitate is 2.9 g. Answer the questions below to determine the percent yield. 3Ca(NO3)2(aq) + 2Na3PO4(aq) → Ca3(PO4)2(s) + 6NaNO3(aq) 1. a. Which product is the precipitate? b. How many moles of the precipitate would one expect to be produced from 0.030 moles of calcium nitrate? c. How many grams of solid do you expect to be produced? d. What is the percent yield?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

You know the right answer?
A student carries out the precipitation reaction shown below, starting with 0.030 moles of calcium n...
Questions

Biology, 23.09.2020 01:01

Biology, 23.09.2020 01:01

Mathematics, 23.09.2020 01:01

Computers and Technology, 23.09.2020 01:01

Mathematics, 23.09.2020 01:01

Mathematics, 23.09.2020 01:01

Mathematics, 23.09.2020 01:01

History, 23.09.2020 01:01

Mathematics, 23.09.2020 01:01

Social Studies, 23.09.2020 01:01


Mathematics, 23.09.2020 01:01

Mathematics, 23.09.2020 01:01

Mathematics, 23.09.2020 01:01


Mathematics, 23.09.2020 01:01


Mathematics, 23.09.2020 01:01