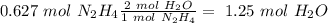Hydrazine, , emits a large quantity of energy when it reacts with oxygen, which has led to hydrazine used as a fuel for rockets: How many moles of each of the gaseous products are produced when 20.1 g of pure hydrazine is ignited in the presence of 20.1 g of pure oxygen

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Hydrazine, , emits a large quantity of energy when it reacts with oxygen, which has led to hydrazine...
Questions

Social Studies, 26.04.2021 08:00


Mathematics, 26.04.2021 08:00

Mathematics, 26.04.2021 08:00

SAT, 26.04.2021 08:00

Mathematics, 26.04.2021 08:00

Mathematics, 26.04.2021 08:00


Physics, 26.04.2021 08:00


Physics, 26.04.2021 08:00

Computers and Technology, 26.04.2021 08:00

English, 26.04.2021 08:00

Mathematics, 26.04.2021 08:00

Mathematics, 26.04.2021 08:00


English, 26.04.2021 08:00



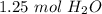 and
and 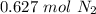
 ) and oxygen (
) and oxygen (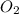 ). So, we can start with the reaction between these compounds:
). So, we can start with the reaction between these compounds: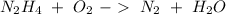
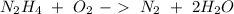
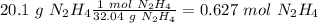
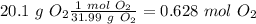
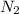 we have a 1:1 molar ratio (1 mol of
we have a 1:1 molar ratio (1 mol of 
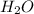 we have a 1:2 molar ratio (2 mol of
we have a 1:2 molar ratio (2 mol of