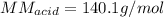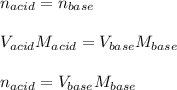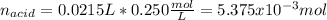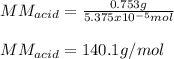Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1

Chemistry, 23.06.2019 16:30
All chemical reactions use reactants in a specific proportion or stoichiometry to form products. the reactant regulates the amount of products produced. a) excess b) limiting c) proportional d) stoichiometric
Answers: 1
You know the right answer?
A 0.753 g sample of a monoprotic acid is dissolved in water and titrated with 0.250 M NaOH. What is...
Questions




Mathematics, 17.05.2021 18:30

Mathematics, 17.05.2021 18:30

Mathematics, 17.05.2021 18:30


Biology, 17.05.2021 18:30

Mathematics, 17.05.2021 18:30


Spanish, 17.05.2021 18:30

History, 17.05.2021 18:30


Mathematics, 17.05.2021 18:30



Mathematics, 17.05.2021 18:30

Mathematics, 17.05.2021 18:30


Mathematics, 17.05.2021 18:30