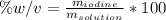Chemistry, 12.08.2020 05:01 Delgadojacky0206
Describe how you would prepare 500ml of 40% (w/v) aqueous iodine solution. [Atomic mass of iodine =127g/mol].

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1
You know the right answer?
Describe how you would prepare 500ml of 40% (w/v) aqueous iodine solution.
[Atomic mass of iodine =...
Questions


Mathematics, 11.01.2021 03:10



English, 11.01.2021 03:10

Mathematics, 11.01.2021 03:10

Mathematics, 11.01.2021 03:10

Mathematics, 11.01.2021 03:10

English, 11.01.2021 03:10

History, 11.01.2021 03:10

Mathematics, 11.01.2021 03:10

Mathematics, 11.01.2021 03:10

Mathematics, 11.01.2021 03:10





Mathematics, 11.01.2021 03:10


Social Studies, 11.01.2021 03:10