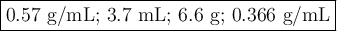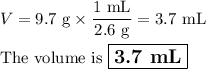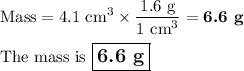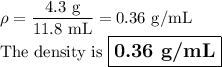Chemistry, 12.08.2020 06:01 naomicervero
Find the density if the volume is 15 mL and the mass is 8.6 g. (5 pts)
Find the volume if the density is 2.6 g/mL and the mass is 9.7 g.(5 pts)
Find the mass if the density is 1.6 g/cm3 and the volume is 4.1 cm3 (5 pts)
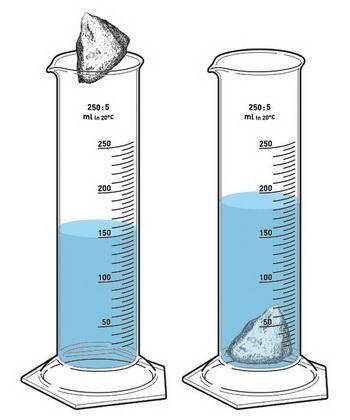
Find the density if the initial volume of water is 12.8 mL, the final volume is 24.6 mL and the mass of the object is 4.3 g. Make a drawing to show the water displacement using a graduated cylinder. (gdoc, gdraw)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2


Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2
You know the right answer?
Find the density if the volume is 15 mL and the mass is 8.6 g. (5 pts)
Find the volume if the densi...
Questions


Mathematics, 23.05.2021 05:20

English, 23.05.2021 05:20



Mathematics, 23.05.2021 05:20


Computers and Technology, 23.05.2021 05:20

Mathematics, 23.05.2021 05:20

History, 23.05.2021 05:20



Mathematics, 23.05.2021 05:20





History, 23.05.2021 05:20

Mathematics, 23.05.2021 05:20