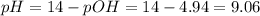Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
Calculate the pH of a solution formed by mixing 250.0 mL of 0.15 M NH4Cl with 200.0 mL of 0.12 M NH3...
Questions

Chemistry, 16.04.2021 17:20



Mathematics, 16.04.2021 17:20




Mathematics, 16.04.2021 17:20

Mathematics, 16.04.2021 17:20

Mathematics, 16.04.2021 17:20

Mathematics, 16.04.2021 17:20

Mathematics, 16.04.2021 17:20


Mathematics, 16.04.2021 17:20

English, 16.04.2021 17:20




Mathematics, 16.04.2021 17:20

English, 16.04.2021 17:20

![[NH_{3}] = \frac{n_{NH_{3}}}{V_{T}} = \frac{C_{i}_{(NH_{3})}*Vi_{NH_{3}}}{V_{NH_{3}} + V_{NH_{4}^{+}}} = \frac{0.12 M*0.2 L}{0.2 L + 0.25 L} = 0.053 M](/tpl/images/0718/8632/272cb.png)
![[NH_{4}^{+}] = \frac{C_{i}_{(NH_{4}^{+})*V_{NH_{4}^{+}}}}{V_{NH_{3}} + V_{NH_{4}^{+}}} = \frac{0.15 M*0.25 L}{0.2 L + 0.25 L} = 0.083 M](/tpl/images/0718/8632/cd416.png)
![Kb = \frac{[NH_{4}^{+}][OH^{-}]}{[NH_{3}]}](/tpl/images/0718/8632/bdcae.png)
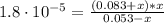
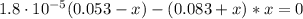
![pOH = -log([OH^{-}]) = -log(1.15 \cdot 10^{-5}) = 4.94](/tpl/images/0718/8632/c67ec.png)