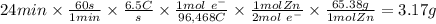Chemistry, 12.08.2020 06:01 sparkybig12
Zinc is used as a coating for steel to protect the steel from environmental corrosion. If a piece of steel is submerged in an electrolysis bath for 24 minutes with a current of 6.5 Amps, how many grams of zinc will be plated out? The molecular weight of Zn is 65.38, and Zn+2 + 2e– → Zn. Question 7 options: A) 3.17 g of Zn B) 1.09 g of Zn C) 6.34 g of Zn D) 12.68 g of Zn

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 07:00
4. glenn andrews recently bought a motorcycle for $3,950. if he had to pay 6% sales tax on the bike, what was the total cost of the motorcycle?
Answers: 1

You know the right answer?
Zinc is used as a coating for steel to protect the steel from environmental corrosion. If a piece of...
Questions



Mathematics, 28.02.2020 04:36

Mathematics, 28.02.2020 04:36



Mathematics, 28.02.2020 04:37


Biology, 28.02.2020 04:37

Advanced Placement (AP), 28.02.2020 04:37


Mathematics, 28.02.2020 04:37