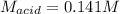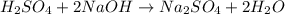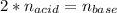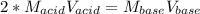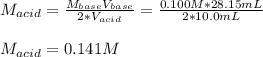Chemistry, 12.08.2020 05:01 KetaFord1978
g If the titration of a 10.0-mL sample of sulfuric acid requires 28.15 mL of 0.100 M sodium hydroxide, what is the molarity of the acid

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Infants born with severe respiratory problems are sometimes given liquid ventilation: they breathe a liquid that can dissolve more oxygen than air can hold. one of these liquids is a fluorinated compound, cf3(cf2)7br. the solubility of oxygen in this liquid is 66 mlo2 per 100 ml liquid. in contrast, air is 21 % oxygen by volume. calculate the moles of o2 present in an infant's lungs (volume: 12 ml ) if the infant takes a full breath of air. assume a pressure of 1 atm in the lungs.
Answers: 1

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
g If the titration of a 10.0-mL sample of sulfuric acid requires 28.15 mL of 0.100 M sodium hydroxid...
Questions



Mathematics, 22.02.2021 07:50




Mathematics, 22.02.2021 07:50


Chemistry, 22.02.2021 07:50

Arts, 22.02.2021 07:50

Mathematics, 22.02.2021 07:50

Mathematics, 22.02.2021 07:50

Chemistry, 22.02.2021 07:50


Mathematics, 22.02.2021 07:50



Advanced Placement (AP), 22.02.2021 07:50