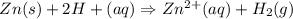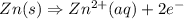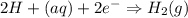Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
A voltaic cell is set up as follows: Anode: Zn electrode in a solution of 0.050 M Zn(NO 3 ) 2 Cathod...
Questions

Mathematics, 12.07.2021 21:20

Mathematics, 12.07.2021 21:20


Social Studies, 12.07.2021 21:20

Chemistry, 12.07.2021 21:20

Mathematics, 12.07.2021 21:20


Mathematics, 12.07.2021 21:20

History, 12.07.2021 21:20


English, 12.07.2021 21:20

Mathematics, 12.07.2021 21:20

Mathematics, 12.07.2021 21:20

Mathematics, 12.07.2021 21:20


Mathematics, 12.07.2021 21:20




Physics, 12.07.2021 21:20