
For the set of ionic compounds, LiCl, AgCl, PbCl2, choose the correct characterization of their solubilities in water from the response list. Group of answer choices All three salts are soluble. None of the three salts are soluble. Two of the three salts are soluble. One of the three salts is soluble.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2


Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2
You know the right answer?
For the set of ionic compounds, LiCl, AgCl, PbCl2, choose the correct characterization of their solu...
Questions

Physics, 29.10.2020 18:40



Social Studies, 29.10.2020 18:40


History, 29.10.2020 18:40

Spanish, 29.10.2020 18:40

Mathematics, 29.10.2020 18:40

Biology, 29.10.2020 18:40

Mathematics, 29.10.2020 18:40




History, 29.10.2020 18:40


Mathematics, 29.10.2020 18:40


History, 29.10.2020 18:40

Mathematics, 29.10.2020 18:40

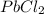 would be insoluble in cold water while LiCl would be soluble.
would be insoluble in cold water while LiCl would be soluble.

