
A chemist prepares a solution of sodium nitrate by measuring out of sodium nitrate into a volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's sodium nitrate solution. Round your answer to 3 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1


Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1
You know the right answer?
A chemist prepares a solution of sodium nitrate by measuring out of sodium nitrate into a volumetric...
Questions





Law, 21.02.2020 01:58






Chemistry, 21.02.2020 01:58



History, 21.02.2020 01:58

Mathematics, 21.02.2020 01:58





Mathematics, 21.02.2020 01:58

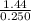 = 5.74M or 5.74 mol/L (to 3 sign. fig.)
= 5.74M or 5.74 mol/L (to 3 sign. fig.)

