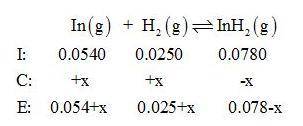Chemistry, 05.08.2020 16:01 XxDonaldTrumpxX452
Gaseous indium dihydride is formed from the elements at elevated temperature:
In(g)+H2(g)⇌InH2(g),Kp=1.48 at 973 K
The partial pressures measured in a reaction vessel are
PIn =0.0540atm
PH2= 0.0250atm
PInH2 =0.0780atm
Calculate Qp and give equal partial pressure for In, H2, and InH2.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1

Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
You know the right answer?
Gaseous indium dihydride is formed from the elements at elevated temperature:
In(g)+H2(g)⇌InH2(g),...
Questions


Biology, 28.10.2021 01:00

Advanced Placement (AP), 28.10.2021 01:00

Biology, 28.10.2021 01:00

World Languages, 28.10.2021 01:00

Mathematics, 28.10.2021 01:00

Biology, 28.10.2021 01:00


History, 28.10.2021 01:00

History, 28.10.2021 01:00



Mathematics, 28.10.2021 01:00

Mathematics, 28.10.2021 01:00

Social Studies, 28.10.2021 01:00

Physics, 28.10.2021 01:00



Social Studies, 28.10.2021 01:00