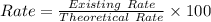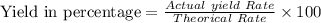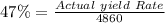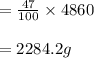Chemistry, 12.08.2020 07:01 hannahmckain
A pharmaceutical company is making a large volume of nitrous oxide (NO). They predict they will be able to make a maximum amount of 4860 grams with the materials they have in stock. From the previous 10 volumes they have made, they know that the percent yield of this reaction is fairly low at 47%. How much will the actual yield be? A. 228 grams B. 2284 grams C. 10340 grams D. 486 grams

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
A pharmaceutical company is making a large volume of nitrous oxide (NO). They predict they will be a...
Questions







Social Studies, 10.06.2020 13:57


Mathematics, 10.06.2020 13:57






Mathematics, 10.06.2020 13:57

Mathematics, 10.06.2020 13:57

Mathematics, 10.06.2020 13:57

Biology, 10.06.2020 13:57

Biology, 10.06.2020 13:57

Mathematics, 10.06.2020 13:57