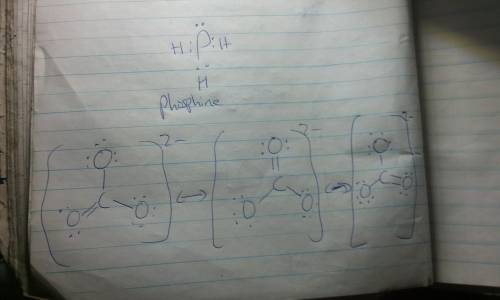
Resonance Structures are ways to represent the bonding in a molecule or ion when a single Lewis structure fails to describe accurately the actual electronic structure. Equivalent resonance structures occur when there are identical patterns of bonding within the molecule or ion. The actual structure is a composite, or resonance hybrid, of the equivalent contributing structures. Draw Lewis structures for thecarbonate ion and for phosphine in which the central atom obeys the octet rule. ... How many equivalent Lewis structures are necessary to describe the bonding in CO32-

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
When the earth was formed and cooled, why did nickel and iron end up in the center of the earth while basalt and granite ended up in the outer layers
Answers: 3

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1
You know the right answer?
Resonance Structures are ways to represent the bonding in a molecule or ion when a single Lewis stru...
Questions







Mathematics, 09.07.2020 02:01







Biology, 09.07.2020 02:01



Computers and Technology, 09.07.2020 02:01