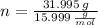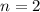Chemistry, 03.08.2020 14:01 ericwheeler821
An organic acid is composed of carbon (68.84%), hydrogen (4.96%), and oxygen (26.20%). Its molar mass is 122.12 g/mol. Determine the molecular formula of the compound.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2


Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
You know the right answer?
An organic acid is composed of carbon (68.84%), hydrogen (4.96%), and oxygen (26.20%). Its molar mas...
Questions


Mathematics, 11.10.2019 17:10

Mathematics, 11.10.2019 17:10





History, 11.10.2019 17:10

History, 11.10.2019 17:10



Engineering, 11.10.2019 17:10

History, 11.10.2019 17:10




Mathematics, 11.10.2019 17:10



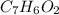 .
.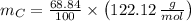
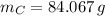
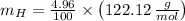
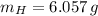
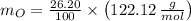
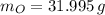
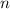 ), measured in moles, of each element are calculated by the following expression:
), measured in moles, of each element are calculated by the following expression: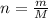
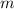 - Mass of the element, measured in grams.
- Mass of the element, measured in grams.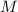 - Molar mass of the element, measured in grams per mol.
- Molar mass of the element, measured in grams per mol.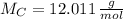 )
)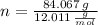
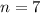
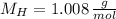 )
)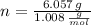
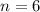
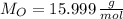 )
)