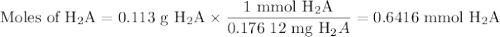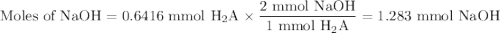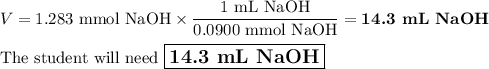Chemistry, 03.08.2020 14:01 lizzie3545
A chemistry student weighs out of ascorbic acid , a diprotic acid, into a volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with solution. Calculate the volume of solution the student will need to add to reach the final equivalence point. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
19. at high pressures, how does the volume of a real gas compare with the volume of an ideal gas under the same conditions, and why? eman- it is much less because real gas partides are not moving. there is no difference because the gas laws are always obeyed. it is much less because at high pressures the temperature drops. it is much greater because real gas partides take up space.
Answers: 1

Chemistry, 21.06.2019 13:20
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins. drag the appropriate molecular formula to their respective bins.
Answers: 3

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 14:30
What is the relationship between wind and ocean waves? question 17 options: wind moving at higher speeds will transfer more energy to the water, resulting in stronger waves. wind moving at higher speeds will transfer energy over a larger part of the ocean water, resulting in waves with a shorter wavelength. winds moving at higher speeds with cause water to move forward at faster rates, causing larger ocean waves. winds moving at higher speeds will affect deeper water, resulting in waves that move at a faster rate. how do temperature and salinity affect deepwater currents? question 15 options: as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
You know the right answer?
A chemistry student weighs out of ascorbic acid , a diprotic acid, into a volumetric flask and dilut...
Questions

Mathematics, 18.09.2020 15:01

Mathematics, 18.09.2020 15:01

Physics, 18.09.2020 15:01

Mathematics, 18.09.2020 15:01

Mathematics, 18.09.2020 15:01

Mathematics, 18.09.2020 15:01

Mathematics, 18.09.2020 15:01

Mathematics, 18.09.2020 15:01

Physics, 18.09.2020 15:01

Chemistry, 18.09.2020 15:01

Mathematics, 18.09.2020 15:01

Mathematics, 18.09.2020 15:01

Social Studies, 18.09.2020 15:01

English, 18.09.2020 15:01

Mathematics, 18.09.2020 15:01

Mathematics, 18.09.2020 15:01

Mathematics, 18.09.2020 15:01

Geography, 18.09.2020 15:01

Mathematics, 18.09.2020 15:01

Mathematics, 18.09.2020 15:01