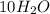Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2
You know the right answer?
n-Butane (C4H10) is burned with stoichiometric amount of oxygen. Determine the mole fraction of carb...
Questions

Mathematics, 05.07.2019 16:00


Biology, 05.07.2019 16:00

Health, 05.07.2019 16:00

Mathematics, 05.07.2019 16:00

Mathematics, 05.07.2019 16:00

Mathematics, 05.07.2019 16:00

Mathematics, 05.07.2019 16:00

Social Studies, 05.07.2019 16:00

English, 05.07.2019 16:00


English, 05.07.2019 16:00

English, 05.07.2019 16:00

English, 05.07.2019 16:00


English, 05.07.2019 16:00



Mathematics, 05.07.2019 16:00

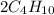 +
+ 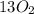 →
→ 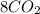 +
+