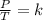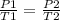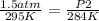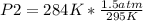Chemistry, 31.07.2020 23:01 saneayahsimmons
Given a fixed amount of gas in a rigid container (no change in volume), what pressure will the gas exert if the pressure is initially 1.50 atm at 22.0oC, and the temperature is changed to 11.0oC?
A. 301 atm
B. 1.56 atm
C. 0.750 atm
D. 1.44 atm
E. 3.00 atm

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

You know the right answer?
Given a fixed amount of gas in a rigid container (no change in volume), what pressure will the gas e...
Questions

Computers and Technology, 05.11.2019 13:31

Biology, 05.11.2019 13:31

Biology, 05.11.2019 13:31

History, 05.11.2019 13:31

Physics, 05.11.2019 13:31

Mathematics, 05.11.2019 13:31



Spanish, 05.11.2019 13:31

Mathematics, 05.11.2019 13:31

Chemistry, 05.11.2019 13:31


Mathematics, 05.11.2019 13:31


Mathematics, 05.11.2019 13:31


Biology, 05.11.2019 13:31