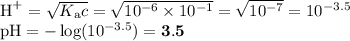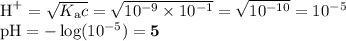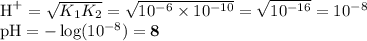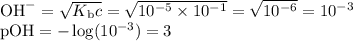Chemistry, 31.07.2020 01:01 seasmarie75
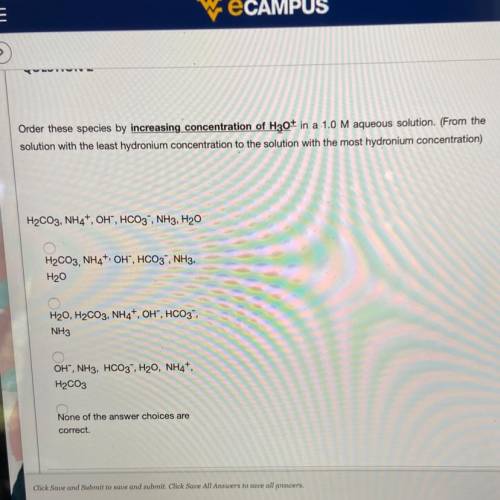
Order these species by increasing concentration of H30+ in a 1.0 M aqueous solution. (From the
solution with the least hydronium concentration to the solution with the most hydronium concentration)
NO
H2CO3, NH4, OH, HCO3, NH3, H20
Home
ir
H2CO3,NH4+, OH", HCO3, NH3,
H20
Paste
H20, H2CO3, NH4+, OH", HCO3-
NH3
6
con
O
OH", NH3, HCO3, H20, NH4+,
H2CO3
None of the answer choices are
correct.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3


Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1
You know the right answer?
Order these species by increasing concentration of H30+ in a 1.0 M aqueous solution. (From the
solu...
Questions


History, 23.07.2019 02:30

History, 23.07.2019 02:30

History, 23.07.2019 02:30


Computers and Technology, 23.07.2019 02:30



Biology, 23.07.2019 02:30

English, 23.07.2019 02:30

Spanish, 23.07.2019 02:30

Biology, 23.07.2019 02:30

English, 23.07.2019 02:30

Biology, 23.07.2019 02:30

Health, 23.07.2019 02:30




Mathematics, 23.07.2019 02:30

History, 23.07.2019 02:30