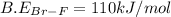Chemistry, 29.07.2020 16:01 brookeboyd7469
Consider the following reaction: Br2(g) + 3 F2(g) LaTeX: \rightarrow→ 2 BrF3(g) LaTeX: \Delta H_{rxn}Δ H r x n= ‒836 kJ/mol Bond Bond Energy (kJ/mol) Br–Br 193 F–F 155 Using the above bond dissociation energies, calculate the energy, in kJ/mol, of a Br–F bond.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3


Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
Consider the following reaction: Br2(g) + 3 F2(g) LaTeX: \rightarrow→ 2 BrF3(g) LaTeX: \Delta H_{rxn...
Questions


English, 31.03.2020 03:04









Mathematics, 31.03.2020 03:04

Social Studies, 31.03.2020 03:04

Mathematics, 31.03.2020 03:04

Computers and Technology, 31.03.2020 03:04






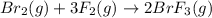
![\Delta H=\sum [n\times B.E(reactant)]-\sum [n\times B.E(product)]](/tpl/images/0714/8206/42942.png)
![\Delta H=[(n_{Br_2}\times B.E_{Br_2})+(n_{F_2}\times B.E_{F_2}) ]-[(n_{BrF_3}\times B.E_{BrF_3})]](/tpl/images/0714/8206/3f797.png)
![\Delta H=[(n_{Br_2}\times B.E_{Br-Br})+(n_{F_2}\times B.E_{F_F}) ]-[(n_{BrF_3}\times 3\times B.E_{Br-F})]](/tpl/images/0714/8206/a662a.png)
![\Delta H=[(1\times 193)+(3\times 155)]-[(2\times 3\times B.E_{Br-F})]](/tpl/images/0714/8206/a838d.png)