Chemistry, 30.07.2020 04:01 jadkins842
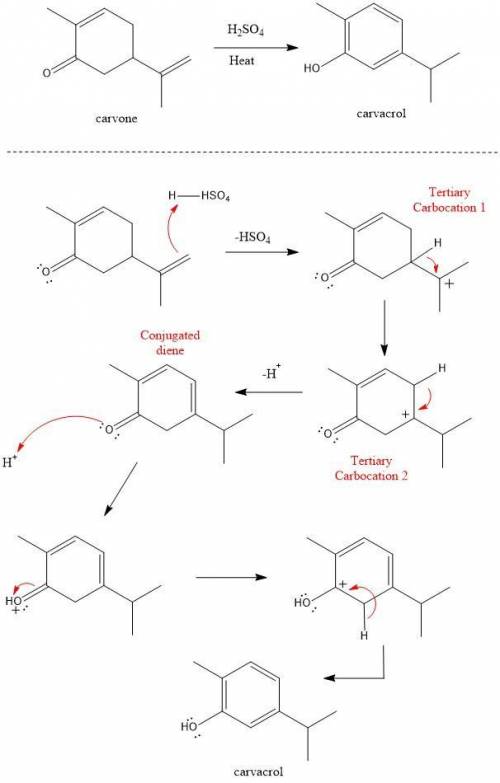
Heating carvone with aqueous sulfuric acid converts it into carvacrol. The mechanism involves the following steps:
1. The terminal alkene of carvone reacts with acid to form tertiary carbocation 1;
2. A hydride shift results in the formation of tertiary carbocation 2;
3. Deprotonation of the ring leads to conjugated diene 3;
4. Deprotonation at the α carbon leads to the product carvacrol.
Required:
Draw the mechanism and then draw the structure of tertiary carbocation 2.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2


Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
You know the right answer?
Heating carvone with aqueous sulfuric acid converts it into carvacrol. The mechanism involves the fo...
Questions

Mathematics, 04.07.2019 22:30






Computers and Technology, 04.07.2019 22:30


Biology, 04.07.2019 22:30

World Languages, 04.07.2019 22:30







Mathematics, 04.07.2019 22:30


Mathematics, 04.07.2019 22:30