Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3


Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
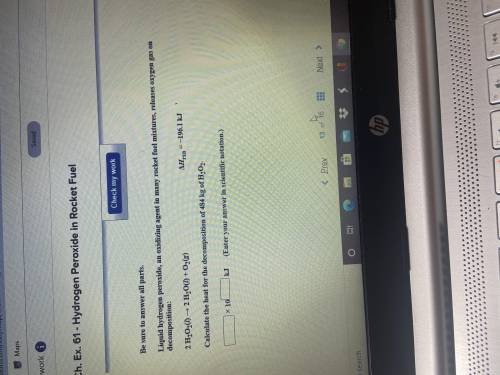
Liquid hydrogen peroxide an oxidizing agent in many rocket fuel mixtures, releases oxygen gas on dec...
Questions

Mathematics, 26.03.2021 17:50



SAT, 26.03.2021 17:50

Biology, 26.03.2021 17:50

Mathematics, 26.03.2021 17:50

Chemistry, 26.03.2021 17:50

Geography, 26.03.2021 17:50





Mathematics, 26.03.2021 17:50

Mathematics, 26.03.2021 17:50


Mathematics, 26.03.2021 17:50



Mathematics, 26.03.2021 17:50

Mathematics, 26.03.2021 17:50