
Chemistry, 28.07.2020 23:01 alexis9658
According to the phase diagram for H20 what happens to the phases of water at 1 atm pressure as the temperature is increased from -10 degrees Celsius to 110 degrees
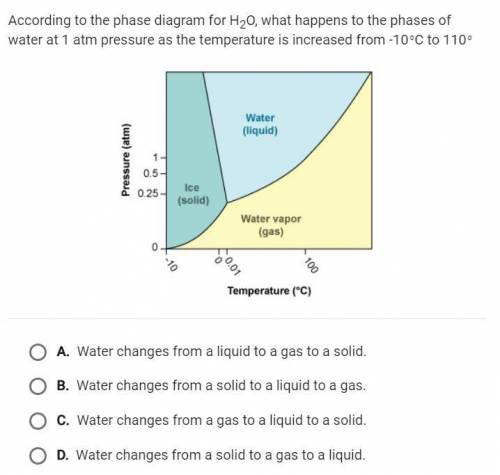

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
According to the phase diagram for H20 what happens to the phases of water at 1 atm pressure as the...
Questions






English, 16.12.2020 17:00

English, 16.12.2020 17:00

Chemistry, 16.12.2020 17:00



History, 16.12.2020 17:00

Mathematics, 16.12.2020 17:00


Mathematics, 16.12.2020 17:00

World Languages, 16.12.2020 17:00







