Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
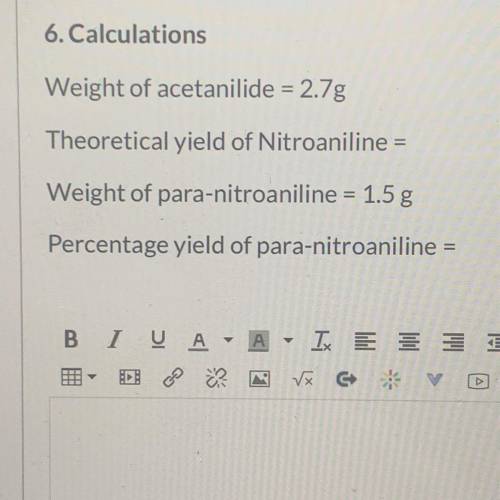
Calculations
Weight of acetanilide = 2.7g
Theoretical yield of Nitroaniline =
Weight of...
Theoretical yield of Nitroaniline =
Weight of...
Questions


History, 13.11.2019 09:31


Mathematics, 13.11.2019 09:31

Mathematics, 13.11.2019 09:31


History, 13.11.2019 09:31

Social Studies, 13.11.2019 09:31





History, 13.11.2019 09:31

History, 13.11.2019 09:31

Mathematics, 13.11.2019 09:31





Business, 13.11.2019 09:31