
Chemistry, 29.07.2020 01:01 drubio102004
A multistep reaction can only occur as fast as its slowest step. Therefore, it is the rate law of the slow step that determines the rate law for the overall reaction. Consider the following multistep reaction:
A+B AB(slow)
A+ABA2B(fast
2A+B A2B(overall)
Based on this mechanism, determine the rate law for the overall reaction.
a) rate = kA2BAB
b) rate = kAB
c) rate = kAAB
d) rate = kA2B

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

You know the right answer?
A multistep reaction can only occur as fast as its slowest step. Therefore, it is the rate law of th...
Questions

Mathematics, 05.02.2021 16:50

Mathematics, 05.02.2021 16:50

Biology, 05.02.2021 16:50



Mathematics, 05.02.2021 16:50

Mathematics, 05.02.2021 16:50

Chemistry, 05.02.2021 16:50

Mathematics, 05.02.2021 16:50


English, 05.02.2021 16:50

Mathematics, 05.02.2021 16:50


Mathematics, 05.02.2021 16:50

Health, 05.02.2021 16:50


Mathematics, 05.02.2021 16:50



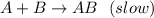
![rate=k[A][B]](/tpl/images/0714/4503/903b2.png)


