
In each of the following groups of substances, pick the one that has the given property. Justify your answer. a) highest boiling point: HBr, Kr, Cl2 b) highest freezing point: H2O, NaCl, Hf c) lowest vapor pressure at 25C: Cl2, Br2, I2 d) lowest freezing point: N2, CO, CO2 e) lowest boiling point: CH4, CH3CH3, CH3CH2CH3 f) highest boiling point: HF, HCl, HBr Could someone help me understand fully how to do this?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

You know the right answer?
In each of the following groups of substances, pick the one that has the given property. Justify you...
Questions

English, 26.12.2020 01:40

Mathematics, 26.12.2020 01:40


Business, 26.12.2020 01:40


English, 26.12.2020 01:40

Mathematics, 26.12.2020 01:40


Mathematics, 26.12.2020 01:40


Health, 26.12.2020 01:40

English, 26.12.2020 01:40

Mathematics, 26.12.2020 02:00

Mathematics, 26.12.2020 02:00

Computers and Technology, 26.12.2020 02:00


Advanced Placement (AP), 26.12.2020 02:00


Mathematics, 26.12.2020 02:00

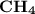 has the lowest molecular weight, thus the lowest freezing point.
has the lowest molecular weight, thus the lowest freezing point.

