
Chemistry, 28.07.2020 05:01 FavvBella84
For the reaction C + 2H2 - CH2, how many moles of hydrogen are required to produce
19.26 mol of methane, CHA?
Select one:
O a. 19.26
O b. 38.52
O c. 15.0
O d. 24.7

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1

Chemistry, 23.06.2019 07:10
1) a light bulb takes in 30 of energy per second. it transfers 3j as use energy. calculate the efficiency. second. it transfers 3j as useful light energy and 27j as heat energy. calculate the efficiency
Answers: 1
You know the right answer?
For the reaction C + 2H2 - CH2, how many moles of hydrogen are required to produce
19.26 mol of met...
Questions


English, 19.11.2020 02:20


Mathematics, 19.11.2020 02:20

Mathematics, 19.11.2020 02:20


Mathematics, 19.11.2020 02:20

English, 19.11.2020 02:20


Engineering, 19.11.2020 02:20


Mathematics, 19.11.2020 02:20

Mathematics, 19.11.2020 02:20

Mathematics, 19.11.2020 02:20

Biology, 19.11.2020 02:20





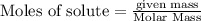
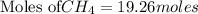
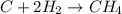
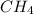 is produced by = 2 moles of
is produced by = 2 moles of 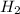
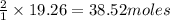 of
of 


