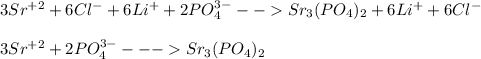Chemistry, 26.07.2020 08:01 njimenez1231
Consider the following precipitation reaction occurring in aqueous solution:
3 SrCl2(aq)+2 Li3PO4(aq) →Sr3(PO4)2(s)+6 LiCl(aq)
Write the complete ionic equation and the net ionic equation for this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:02
If 0.414 g of hydrogen is obtained in this experiment how many grams of sulfur must be obtained
Answers: 2


Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
You know the right answer?
Consider the following precipitation reaction occurring in aqueous solution:
3 SrCl2(aq)+2 Li3PO4(a...
Questions

Social Studies, 15.07.2020 01:01





Physics, 15.07.2020 01:01





English, 15.07.2020 01:01




Mathematics, 15.07.2020 01:01