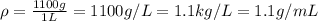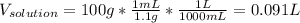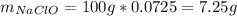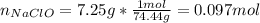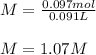Chemistry, 27.07.2020 01:01 santana647
1L of bleach has a mass of 1,100 grams, 7.25% of the mass of bleach is NaClO, 1 mol of NaClO has a mass of 74.44 grams. What is the molarity (mol/L) of NaClO in the bleach? A.0.097 B.0.93 C. 1.07 D.79.75

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
1L of bleach has a mass of 1,100 grams, 7.25% of the mass of bleach is NaClO, 1 mol of NaClO has a m...
Questions


Mathematics, 05.05.2021 17:50

Mathematics, 05.05.2021 17:50



Chemistry, 05.05.2021 17:50

Mathematics, 05.05.2021 17:50



Mathematics, 05.05.2021 17:50

Mathematics, 05.05.2021 17:50



Computers and Technology, 05.05.2021 17:50

Computers and Technology, 05.05.2021 17:50

Social Studies, 05.05.2021 17:50

English, 05.05.2021 17:50

World Languages, 05.05.2021 17:50

Mathematics, 05.05.2021 17:50