
Chemistry, 27.07.2020 01:01 tammydbrooks43
1. What is the net ionic equation for the reaction that occurs when aqueous solutions of AgNO3 and CaCl2 are mixed and a precipitate forms? A. Ca+2(aq) + NO3-(aq) Ca(NO3)2(aq) B. Ag2+(aq) + 2Cl-(aq) AgCl2(s) C. Cl−(aq) + Ag+(aq) ⟶ AgCl(s) D. None of the above because no reaction occurs

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
1. What is the net ionic equation for the reaction that occurs when aqueous solutions of AgNO3 and C...
Questions

Social Studies, 18.07.2021 16:40

Mathematics, 18.07.2021 16:40

Physics, 18.07.2021 16:40


Advanced Placement (AP), 18.07.2021 16:40

Social Studies, 18.07.2021 16:40

English, 18.07.2021 16:50

Physics, 18.07.2021 16:50




History, 18.07.2021 17:00

Mathematics, 18.07.2021 17:00

Mathematics, 18.07.2021 17:00


Mathematics, 18.07.2021 17:00

Mathematics, 18.07.2021 17:00


Mathematics, 18.07.2021 17:00

English, 18.07.2021 17:00

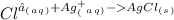
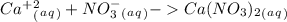
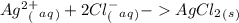
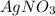 are:
are: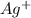 and
and 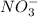
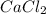 are:
are: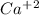 and
and 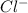
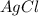 and
and 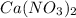
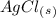 and an aqueous state for
and an aqueous state for 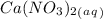 .
. 

