

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1
You know the right answer?
The equilibrium constants for the chemical reaction N 2(g) + O 2(g) 2NO(g) are K P = 1.1 × 10 –3 and...
Questions


Mathematics, 26.02.2021 21:50

History, 26.02.2021 21:50

Mathematics, 26.02.2021 21:50



English, 26.02.2021 21:50



Mathematics, 26.02.2021 21:50

Mathematics, 26.02.2021 21:50

Mathematics, 26.02.2021 21:50



Biology, 26.02.2021 21:50


Chemistry, 26.02.2021 21:50


Arts, 26.02.2021 21:50

English, 26.02.2021 21:50

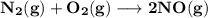
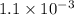
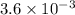
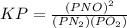
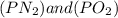 is defined in the denominator section and the value of
is defined in the denominator section and the value of 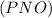 is defined in the numerator section that defines the value of KP is increases.so, the temperature of the KP will be KP ∝ (PNO).
is defined in the numerator section that defines the value of KP is increases.so, the temperature of the KP will be KP ∝ (PNO).

