
Chemistry, 26.07.2020 14:01 sarahhN7534
0.60 atm of SO3 and 0.30 atm of SO2are placed in a container and the system is allowed to reach equilibrium. Calculate the pressure of O2(g) at equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
You know the right answer?
0.60 atm of SO3 and 0.30 atm of SO2are placed in a container and the system is allowed to reach equi...
Questions

Mathematics, 06.07.2019 07:00


English, 06.07.2019 07:00

Biology, 06.07.2019 07:00

Mathematics, 06.07.2019 07:00


Mathematics, 06.07.2019 07:00

Social Studies, 06.07.2019 07:00

Physics, 06.07.2019 07:00

History, 06.07.2019 07:00

Mathematics, 06.07.2019 07:00

Health, 06.07.2019 07:00

Mathematics, 06.07.2019 07:00

Mathematics, 06.07.2019 07:00

Mathematics, 06.07.2019 07:00

Mathematics, 06.07.2019 07:00

Geography, 06.07.2019 07:00

Mathematics, 06.07.2019 07:00

Mathematics, 06.07.2019 07:00

English, 06.07.2019 07:00

![[O_2] = 4.8 *10^{-5} \ atm](/tpl/images/0713/4227/83355.png)
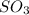 is
is ![[SO_3 ] = 0.63 \ atm](/tpl/images/0713/4227/96504.png)
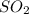 is
is ![[SO_ 2] = 0.30 \ atm](/tpl/images/0713/4227/bdacf.png)
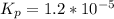
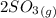 ⇔
⇔ 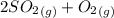
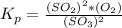
![[O_2] = \frac{k_p * [SO_3] ^2 }{[SO_2]^2}](/tpl/images/0713/4227/a8504.png)
![[O_2] = \frac{1.2 *10^{-5} * 0.60 ^2 }{0.30^2}](/tpl/images/0713/4227/296ec.png)



