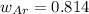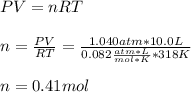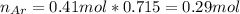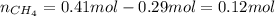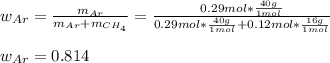Chemistry, 26.07.2020 01:01 jaredsangel08
A 10.0 L flask at 318 K contains a mixture of Ar and CH4 with a total pressure of 1.040 atm. If the mole fraction of Ar is 0.715, what is the mass percent of Ar?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
A 10.0 L flask at 318 K contains a mixture of Ar and CH4 with a total pressure of 1.040 atm. If the...
Questions

Health, 25.08.2019 09:20

English, 25.08.2019 09:20




Chemistry, 25.08.2019 09:20

Social Studies, 25.08.2019 09:20


Social Studies, 25.08.2019 09:30

Chemistry, 25.08.2019 09:30

English, 25.08.2019 09:30

Biology, 25.08.2019 09:30

Geography, 25.08.2019 09:30



History, 25.08.2019 09:30

History, 25.08.2019 09:30

History, 25.08.2019 09:30

English, 25.08.2019 09:30

Geography, 25.08.2019 09:30