Which profile best describes the reaction C(s) + 2H2(g) → CH4(9),
AH = -74.9 kJ?
...

Chemistry, 25.07.2020 01:01 mrstealyogirl40
Which profile best describes the reaction C(s) + 2H2(g) → CH4(9),
AH = -74.9 kJ?
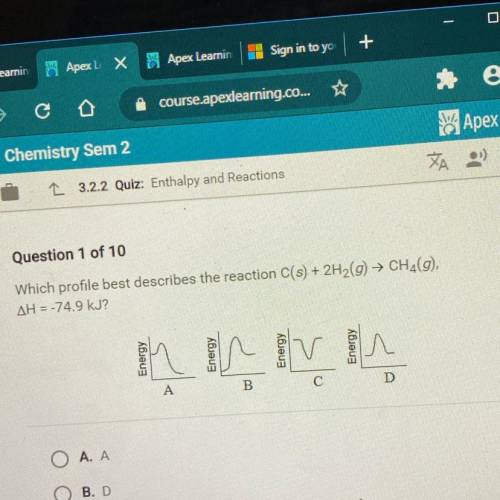

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
Questions


English, 01.10.2019 01:50





Biology, 01.10.2019 01:50



History, 01.10.2019 01:50

History, 01.10.2019 01:50

History, 01.10.2019 01:50




Mathematics, 01.10.2019 01:50

Mathematics, 01.10.2019 01:50


Physics, 01.10.2019 01:50

Mathematics, 01.10.2019 01:50



